
Figure | China Network
Source | Cybertron Equipment
Author | Qinjiu Dongtai
On the afternoon of November 29th, the press conference of the State Council Joint Defense and Control Mechanism was held. National Health Commission responded to the recent incidents in which nucleic acid testing institutions were seriously dealt with because of fraud and other acts:
"For nucleic acid testing, we have always strictly controlled the access and quality of testing qualifications, constantly optimized technical specifications, and focused on strengthening the supervision of testing institutions, including third-party testing institutions. Since the beginning of this year, the health administrative departments in Beijing, Hefei, Anhui, Shijiazhuang, Hebei, Xuchang, Henan, Inner Mongolia and other places have found some illegal problems in the supervision of testing institutions, and all of them have been severely punished. Some illegal institutions and individuals have also been investigated for criminal responsibility.
In the next step, we will continue to strengthen supervision, and resolutely deal with serious violations of the law by issuing false test reports. "
In the past few days, nucleic acid detection has been caught in the whirlpool of public opinion, while Shenzhen nuclear gene is the whirlpool in the whirlpool.
01 Constant violations and "expansion"?
On November 25th, a notice from Lanzhou Health and Health Commission became the focus of public opinion.
According to the notification, at 1 o’clock on November 24, the staff of Lanzhou Nuclear Huaxi Laboratory mistakenly entered the list information of individuals with abnormal nucleic acid testing into the negative personnel information package and uploaded it to the work system, so that the health code of individual persons to be transported showed negative nucleic acid testing. Lanzhou Health and Health Commission said that Lanzhou Nuclear Huaxi Laboratory will be dealt with seriously.

Enterprise investigation shows that this Lanzhou Nuclear Huaxi Laboratory (hereinafter referred to as "Lanzhou Huaxi") was established just over three months ago. Just over a year ago, Lanzhou Huaxi’s brother company, Jinan Huaxi Medical Inspection Co., Ltd. (hereinafter referred to as "Jinan Huaxi"), was notified by the Health and Health Commission of Xingtai City, Hebei Province for its nucleic acid detection fraud.
It has been observed that since 2020, laboratories related to nuclear Hua Xi have been punished many times.
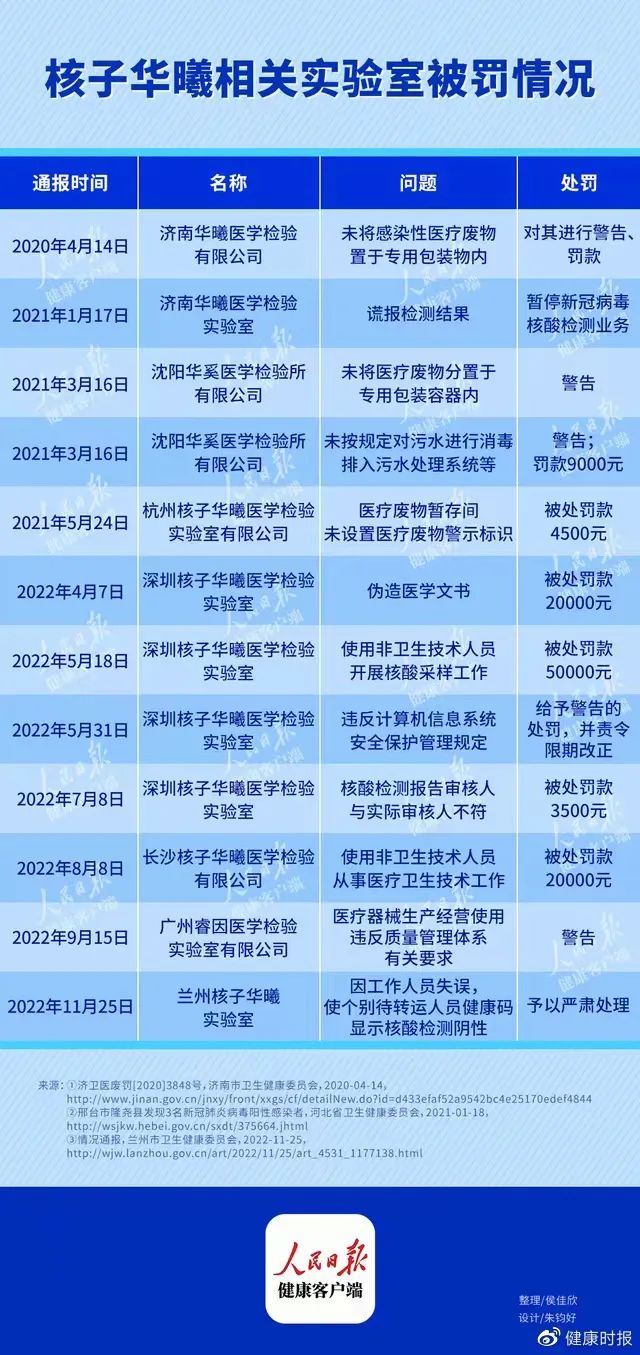
The parent company of these involved companies is Shenzhen Nuclear Gene Technology Co., Ltd. (hereinafter referred to as Shenzhen Nuclear Gene), and the founder is Zhang Nuclear. According to the industrial and commercial data, the actual controllers of more than 30 medical inspection laboratories under the Nuclear Gene are all Zhang Hezhen, and Zhang Shanshan is the supervisor of more than 10 of them.
Since 2022, 16 medical laboratories have been opened for nuclear genes, 8 of which were established in October.
According to "Nuclear Gene Group" WeChat official account, in 1990, Zhang He was admitted to China Medical University and became the first batch of DNA identification graduate students in China. After graduation, shenzhen public introduced high-tech talents to shenzhen public to organize the earliest DNA identification center in China.
After leaving the Public Security Bureau, he started his own business and set up Shenzhen Hongshi Villa Decoration Company. In 2012, Shenzhen Nuclear Gene Technology Co., Ltd. was established. In 2013, Zhang Nuclear established Guangdong Huaxi Forensic Material Evidence Judicial Appraisal Institute, and established the first laboratory with the qualification of judicial appraisal license.
Zhang Hezhen once said: "There are many companies doing genetic testing now, but there are actually only two types of companies in the market today, 3% are reliable, 97% are unreliable, and I only do 3%."
02 The track is "overcrowded" and accounts receivable have increased substantially.
An epidemic situation made the subdivision track of nucleic acid detection instantly become a crowded "square", and the detection level became uneven under the crowd.
According to the incomplete statistics of People’s Daily Health Client, at least 10 nucleic acid detection institutions in 7 places have been put on file for investigation, notification or punishment this year, involving Beijing, Hefei, Anhui, Shijiazhuang, Hebei, Xuchang, Henan, Hohhot, Inner Mongolia, Kunming, Yunnan and Lanzhou, Gansu.
Since June this year, documents have been issued continuously at the national level to strengthen the supervision of nucleic acid detection and ensure the accuracy of test results. On June 2, the State Council Joint Prevention and Control Mechanism issued the Notice on Further Strengthening the Whole Chain Supervision of Nucleic Acid Testing in Covid-19, which will strictly manage the qualifications of testing institutions and personnel, standardize the management of sample collection, preservation and transshipment, strengthen the daily supervision and management of nucleic acid testing institutions, strengthen the supervision of nucleic acid testing institutions in emergency and other aspects, and directly revoke the Practice License of Medical Institutions by setting up a "traffic light" for the entry and exit mechanism of nucleic acid testing institutions.
On June 7th, National Medical Products Administration issued a notice to further strengthen the quality and safety supervision of detection reagent products in Covid-19 to ensure product quality and safety and effectively serve the overall situation of epidemic prevention and control.
The sudden "expansion" of the track stems from the attraction of huge demand.
Since 2020, as an important member to undertake the task of nucleic acid detection, the status of third-party medical testing institutions has greatly jumped, and the industry has undergone earth-shaking changes.
According to the statistics of orient securities, the market scale of nucleic acid detection in China will reach 13.2 billion yuan in 2021, with a year-on-year increase of +9.1%. According to the forecast of CBinsights, the market scale is expected to reach 14.6 billion yuan in 2022, showing a rapid growth trend. According to the data of enterprise investigation, there are 1702 enterprises related to medical testing in China, including about 145 new enterprises related to medical testing this year, and the investment of enterprises continues to increase.
Taking the head enterprise as an example, in the first three quarters of 2022, Dean Diagnostics realized revenue of 15.63 billion yuan, a year-on-year increase of 67.37%; The net profit of returning to the mother was 2.428 billion yuan, a year-on-year increase of 96.94%. The substantial increase in performance is mainly due to the growth of inspection business.
The data shows that the diagnostic service business grew rapidly, with revenue of 9.875 billion yuan, up 117% year-on-year, and revenue excluding COVID-19 business was 3.485 billion yuan, up 20% year-on-year. It can be seen that COVID-19’s business growth is obviously faster than that of regular business, which is an important driving factor for revenue growth.
Compared with 2019, the annual revenue of Dean Diagnostics was 8.453 billion yuan, and the net profit returned to the mother was 347 million yuan. After the epidemic "Black Swan" flew in, the figures on Dean’s report card of diagnostic profit have multiplied several times on the basis of a larger base.
Another leading enterprise, Jinyu Medicine, also achieved a large performance growth this year. In the first three quarters of 2022, the operating income of Jinyu Medical was 12.208 billion yuan, a year-on-year increase of 41.67%; The net profit of returning to the mother was 2.448 billion yuan, a year-on-year increase of 46.41%. The increasing demand for laboratory tests is also the main reason for the rapid growth of Jinyu medical business.
But behind the 10 billion revenue, the real data is not as optimistic as it seems.
Relevant performance reports show that as of the end of September this year, Dean’s diagnosed accounts receivable rose to 10.754 billion yuan, a year-on-year increase of 71.02%. The accounts receivable of Jinyu Medical also reached 7.433 billion yuan, accounting for 60% of the revenue in the same period.
From the perspective of accounts receivable accounting for total assets, in 2019, Dean diagnosed that accounts receivable accounted for 30.98% of total assets, and in the first half of 2022, accounts receivable accounted for 51.15% of total assets.
It has been observed that the sharp increase of accounts receivable widely exists in enterprises that operate COVID-19 detection related reagents and provide nucleic acid detection services.
Many enterprises responded that the status quo of accounts receivable basically matched the characteristics of business model and the growth of income scale. Because accounts receivable mainly came from medical institutions and the government, the risk of bad debts was low and the overall risk of bad debts was controllable. However, there are also media reports that some enterprises are under greater pressure to pay back the money, and a few small enterprises have greater cash flow risks.
In addition to the income from testing in COVID-19, the routine business of head enterprises has also achieved a certain degree of growth in the past two years. In the first half of 2022, the revenue from the diagnosis service business excluding COVID-19 business of Dean Diagnostics was 4.014 billion yuan, an increase of 31.97% compared with the same period of last year and 37.69% compared with the same period of 2019.
However, it is an obvious fact that the original conventional market for nucleic acid detection cannot bear such a large scale of business. When it returns to normal, the existing market scale will shrink sharply, and the huge production capacity and personnel blindly expanded due to the epidemic will be difficult to digest and absorb.
Once the lucrative scenery is unsustainable, how to "land" is the biggest problem facing these enterprises.
- END –
关于作者